Digital Health Outcomes Blog
The Academy of Managed Care Pharmacy (AMCP) and Digital Health Outcomes are pleased to announce a strategic partnership on AMCP eModel.
During the meeting we are introducing AMCP eModel – a new framework to improve utility, transparency and usefulness of health economic models. “The Academy of Managed Care Pharmacy (AMCP) and Digital Health Outcomes (DHO) are pleased… Read more
Meet Digital Health Outcomes and Global Market Access Solutions at ISPOR Europe 2023
The Digital Health Outcomes Team will be attending ISPOR Europe 2023 in Copenhagen. We are looking forward to meet old friends and explore new connections! If you’d like to meet and understand how Digital Health Outcomes… Read more
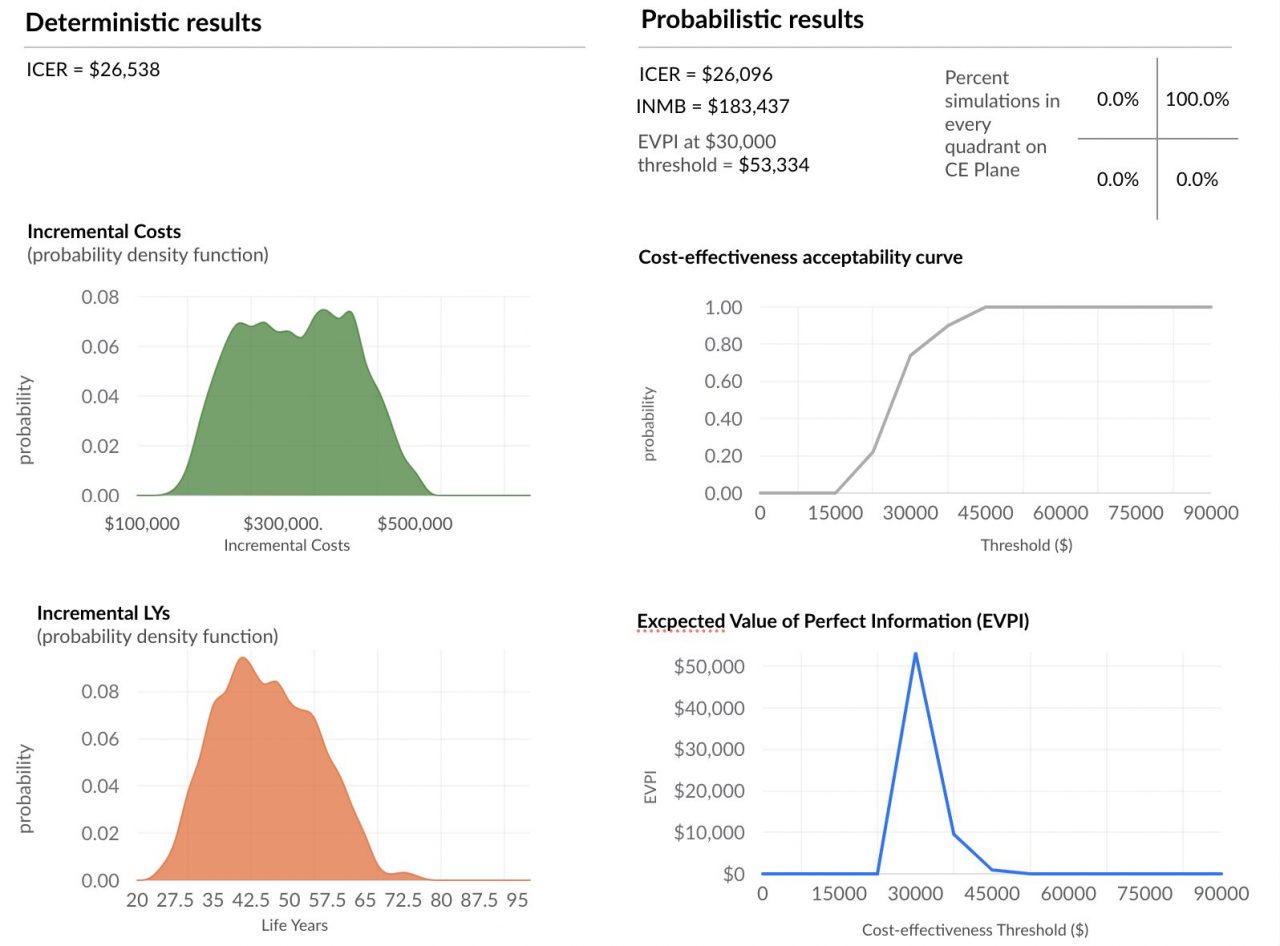
Much faster probabilistic sensitivity analysis (PSA) with cloud based horizontal autoscaling
Background and definitions: Decision-makers require estimates of decision uncertainty alongside expected net benefits (NB) of interventions. This requirement may be difficult in computationally expensive models, for example, those employing patient level simulation[1]. Probabilistic sensitivity analysis (PSA) is… Read more
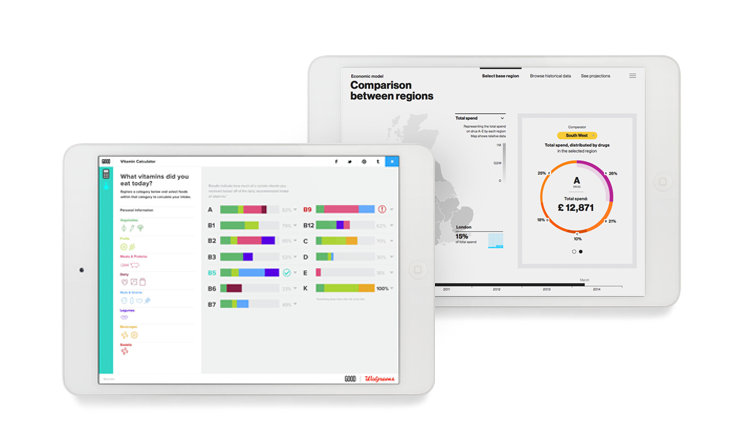
Interactive health data visualization approaches: good practices and examples
How interactive data visualizations change communication of health data Presentation of health data is undergoing a big change, one that will influence not only how payers and providers make their decisions but also how pharma, medical… Read more
Automatic Data Sourcing for Health Economic Models – Interoperable data for timely decision-making
This article is inspired by the exciting idea of data interoperability in health economics modelling process, which means sourcing (fetching) inputs for models in real-time, using modern software infrastructure capabilities. On a larger scale, development of… Read more
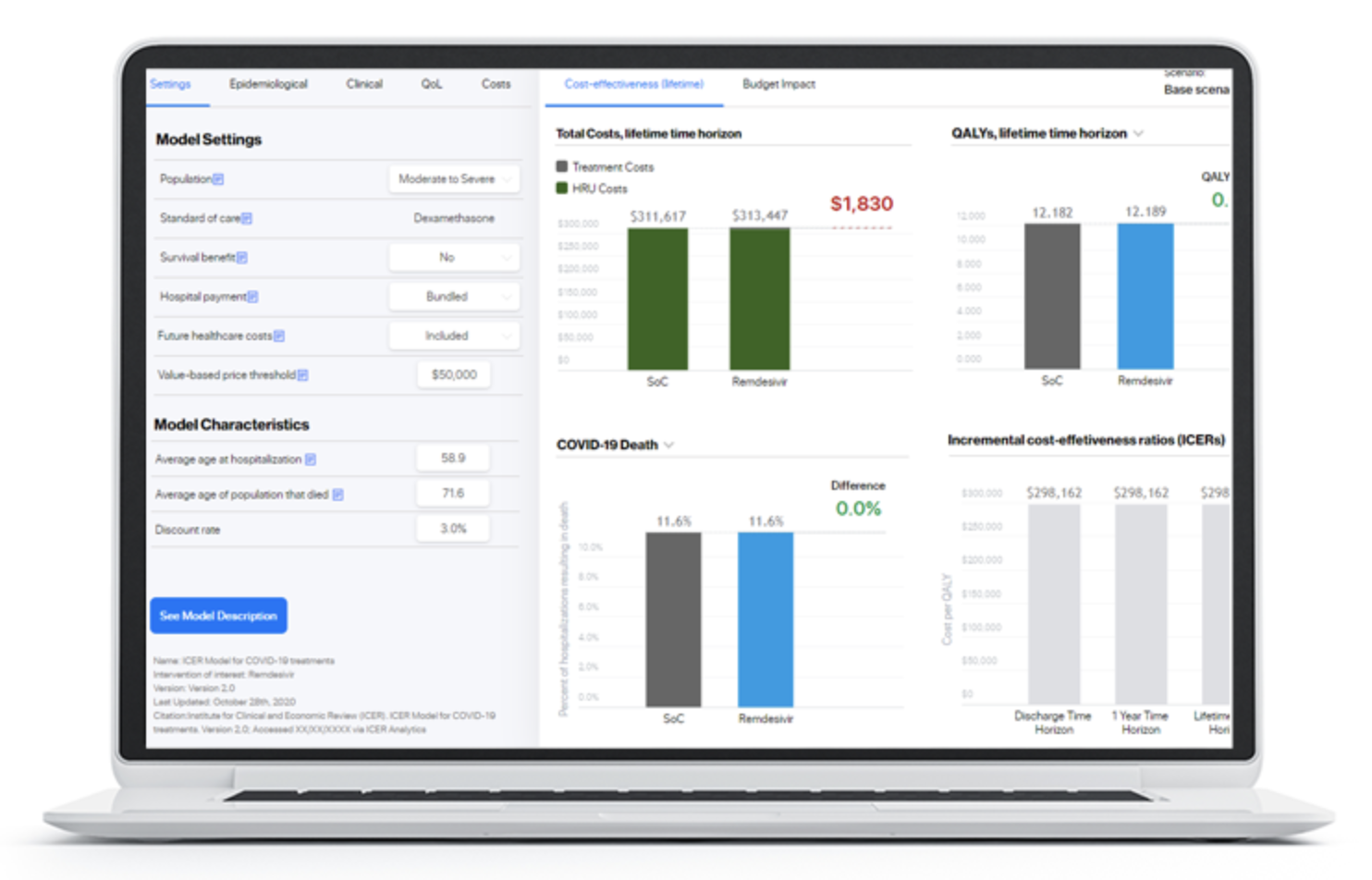
Digital Health Outcomes launched interactive health economic modeling platform together with the Institute for Clinical and Economic review (ICER).
We are delighted to support the US based Institute for Clinical and Economic (ICER) in launching a dedicated interactive modeling platform hosting a wide variety of cost-effectiveness and budget impact models developed by ICER and their… Read more
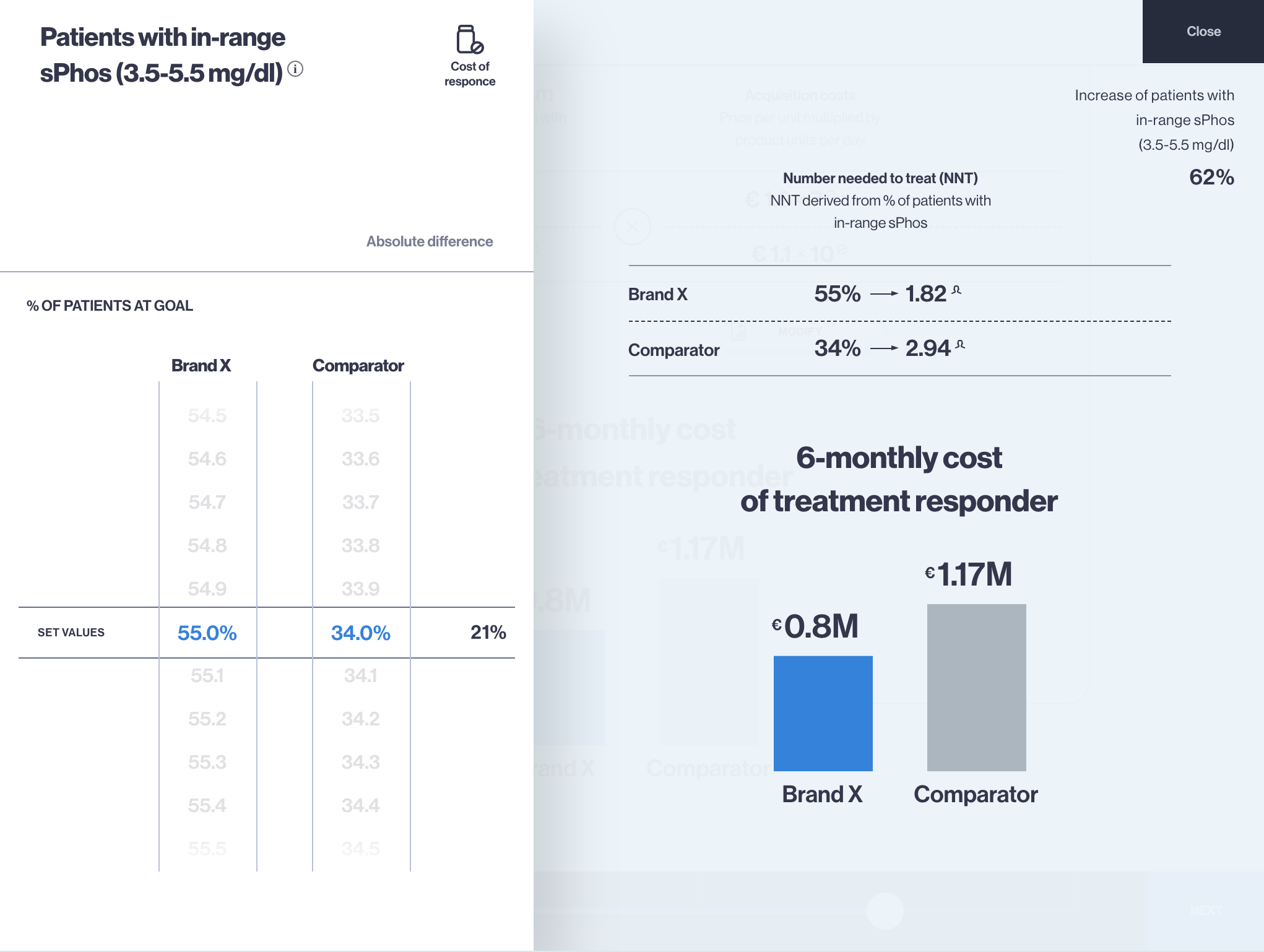
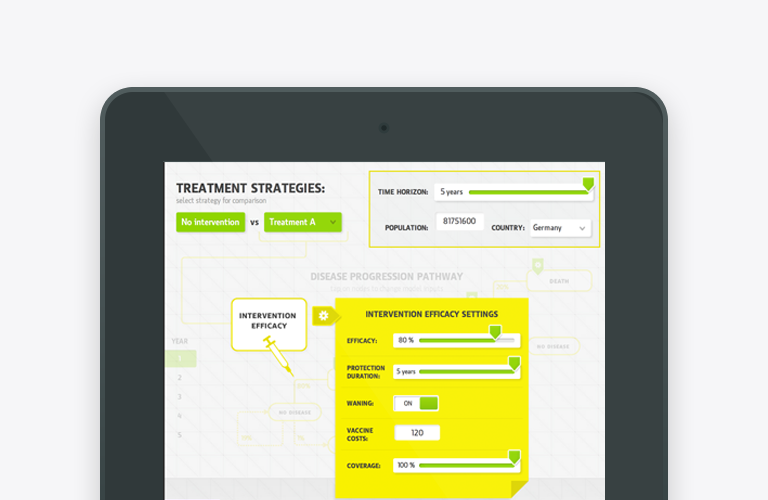
Budget Impact Model app for iPad and Web – an effective tool for payer engagement
Budget Impact Model (BIM) is the health economics type of model which calculates the net cost of including a new drug or therapy into certain healthcare system or payer, hospital settings. Budget impact model app (budget… Read more
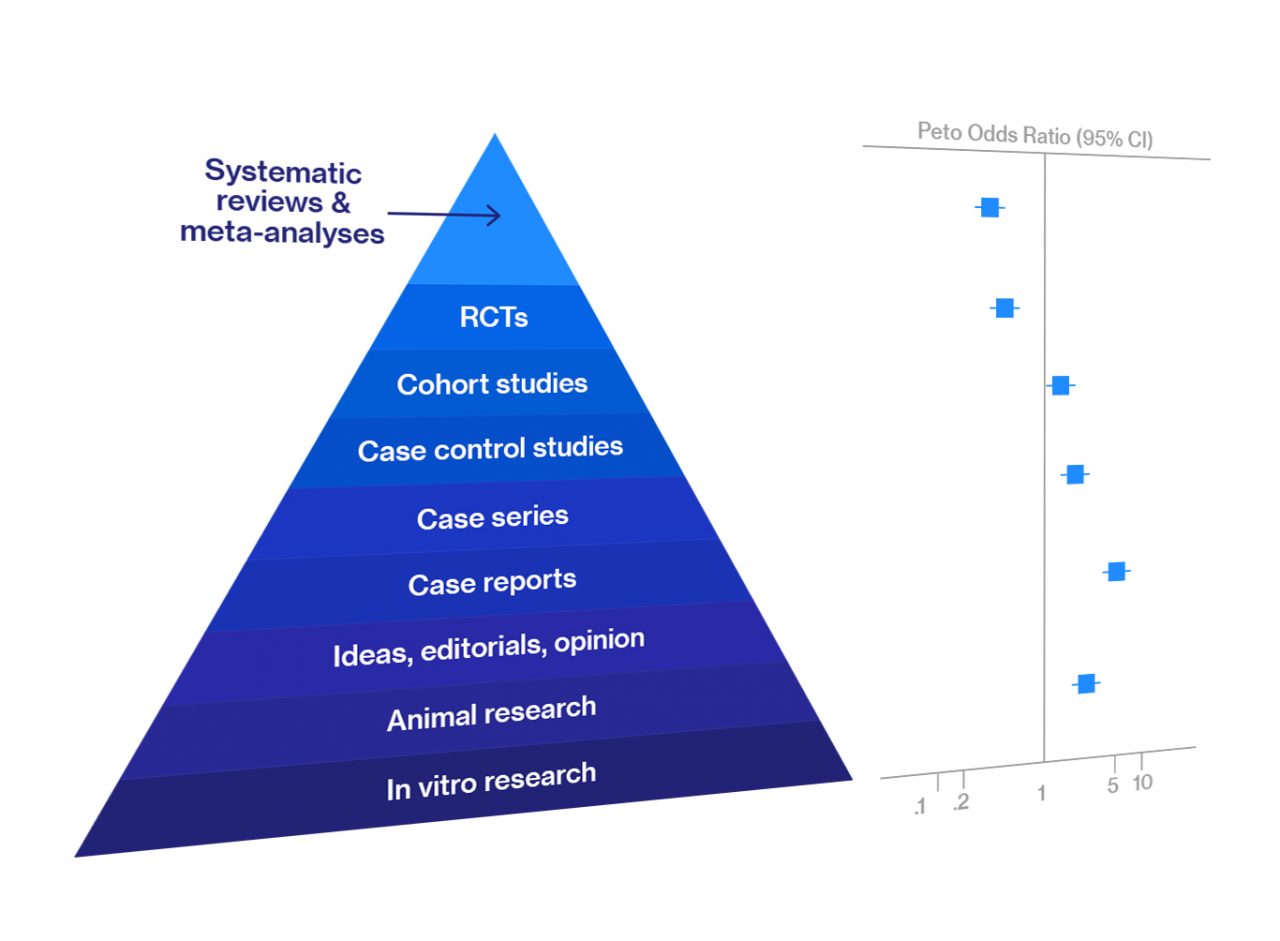
Systematic Review and Meta-Analysis — Overview
Meta-analysis and systematic review are at the top of the hierarchy of evidence for data reliability in various types of biomedical research. They are an integral part of evidence-based medicine. Most clinicians begin searching for the best, evidence-based… Read more
mClinical Research – compliant mobile applications that fit each study protocol
Mobile apps and study portals with CFR 21 Part 11 compliance, infrastructure, and process, developed specifically for the biopharma R&D organisation. Meet us at ISPOR in Boston to learn more about our mClinical Research software and… Read more
What is Real World Evidence (RWE) and Real World Data (RWD)?
The aim of real-world evidence (RWE), is to improve healthcare decision-making by complementing data generated from randomized controlled trials (RCTs). While RCTs come in handy to investigate the question ‘Can this health technology work?’, RWE is… Read more