HEOR or Health Economics and Outcomes Research – term and job position/function used in the pharmaceutical industry. It is also an emerging field of research in health economics that focuses on providing pharmaceutical and life sciences companies with evidence of the value of a new medicine. It establishes and measures the relationship between the treatment process, the costs and the outcomes. It uses data sources of various kinds:
- Clinical trial results.
- Real World Data (RWD) and actual results from a medical practice based on heterogenous patient population
- Financial calculations, price comparisons, and other market indicators.
- Indicators of quality of life and patient satisfaction after a course of treatment.
The results of HEOR and HTA studies can improve treatment or care protocols. They are substantiated by statistical evidence, which in turn lead to qualitative improvements in population health. They evaluate financial resources and the important notion of opportunity costs in generating health in a society. Economic theory implies utilisation maximisation through improvements in allocative efficiency by selecting interventions that provide the best value for money. In other words HEOR research is the practical application of economics to health care in order to identify the optimal treatment in terms of efficiency and cost.
Health Economics and Outcomes Research (HEOR) is also an area of economic analysis that deals with finding data to feed simulation models based on available clinical (ex. meta-analysis), epidemiological, economic and high GRADE published evidence and data to inform rational and evidence-based decision making.
What are the objectives of HEOR consulting?
HEOR consulting companies support clients with developing economic models to demonstrate pharmacoeconomic value for healthcare payers and providers. They usually build models, conduct systematic literature reviews, collect data by performing epidemiological studies to support economic model parameters.
HEOR data are used to inform and make rational, evidence-based decisions related to health care and health services:
- Achieving market access.
- Treatment reimbursement.
- Pricing.
- Contracting with health care providers and insurance companies.
HEOR consultants and researchers try to get answers to these kinds of questions:
- The relationship between the cost and economic value of the medication.
- Is the drug affordable and what are cost the cost-saving and effective scenarios.
- What is the level of uncertainty around point estimates of incremental cost-effectiveness ratios and budget impact.
Taking into account that the majority of HEOR efficacy data are taken from clinical trials, Real World Data could more precisely determine the real effectiveness of drugs and procedures and complement clinical trials.
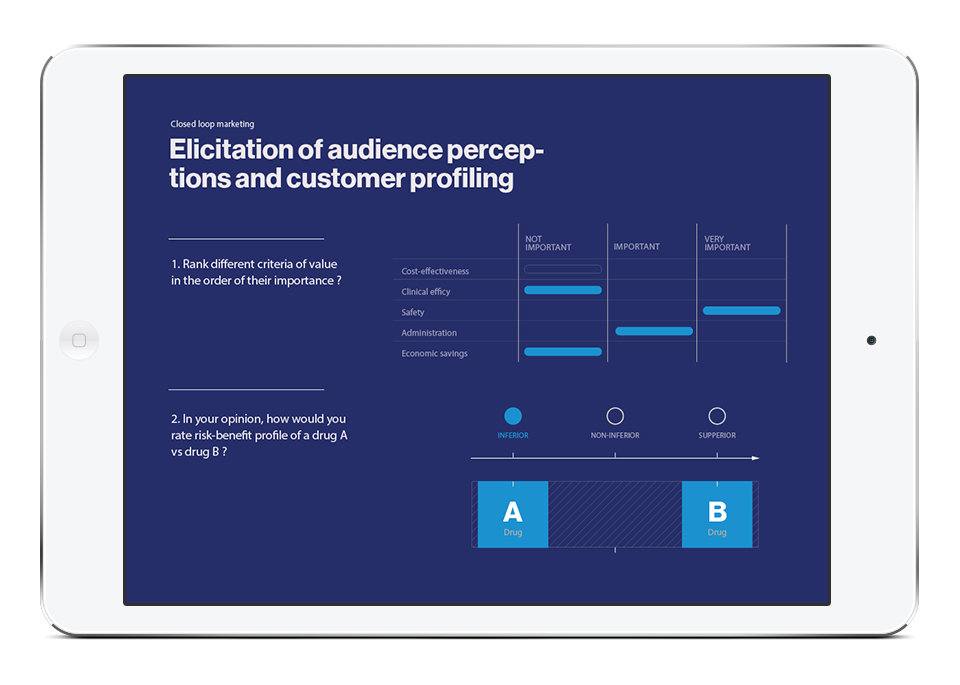
Also, the results allow experts in the field of global health economics and outcomes research to determine how much more valuable a new drug is than existing ones on the market. In this case, the value is understood as the ratio of its cost, effectiveness and affordability for payers.
“The main aim of determining affordability in the health care sector is to evaluate whether the expenses of the intervention can be met. In addition to an intervention’s effectiveness, decision-making requires consideration of the intervention’s feasibility, sustainability and affordability. Affordability also tells us something about the value of alternative health-care services. While considering affordability, it is important to take into account all possible costs and consequences of an intervention.”
Reference: Glossary of HTA Adaptation-Terms
What kind of companies need HEOR modelling and analysis?
Healthcare payers (government, insurance companies, hospitals) operating under constrained budgets are always seeking to maximise the output (value, health, procedures) subject to limited financial resources and other constraints (technical, time, legal, management changes), thus Health Economics and Outcomes Research is in increasing demand.
HEOR research and modelling are mainly conducted by pharmaceutical and equipment manufacturing companies. HEOR data play an important role in developing the company’s market access approach for the development and promotion of its products. Also, the data obtained from the research will be useful to:
- Companies that sell medications, supplements, and medical devices.
- Medical service providers (private and public hospitals, clinics and other institutions).
- Health insurance companies.
- Government agencies and institutions are responsible for healthcare decision-making.
HEOR research for pharmaceutical manufacturers
HEOR research for drug manufacturers during the product development and commercialisation process includes:
- Understand available data. During the research process, experts identify data gaps that serve as evidence of a product’s value and develop a plan for additional research that enables it.
- Develop early economic models. Enable calculations and projections needed to achieve market access and reimbursement from time of product launch.
- Analysing data from clinical trials to build product value. Understands how clinical data (risk ratios) can be translated into health improvements and survival benefits as described by the health economic model.
- Synthesis and analysis of data from published studies. Selecting high-grade evidence is important to achieve robustness in the estimates of economic value.
- Development of cost-effectiveness and budget impact models. Calculations derived from such models are used in the pricing process and in payer reimbursement negotiations.
- Real-world data collection. HEOR firm research involves developing and conducting analyses of real-world data generated from the use of a product after its launch. The results of the analysis help increase the value of the product throughout its lifecycle.
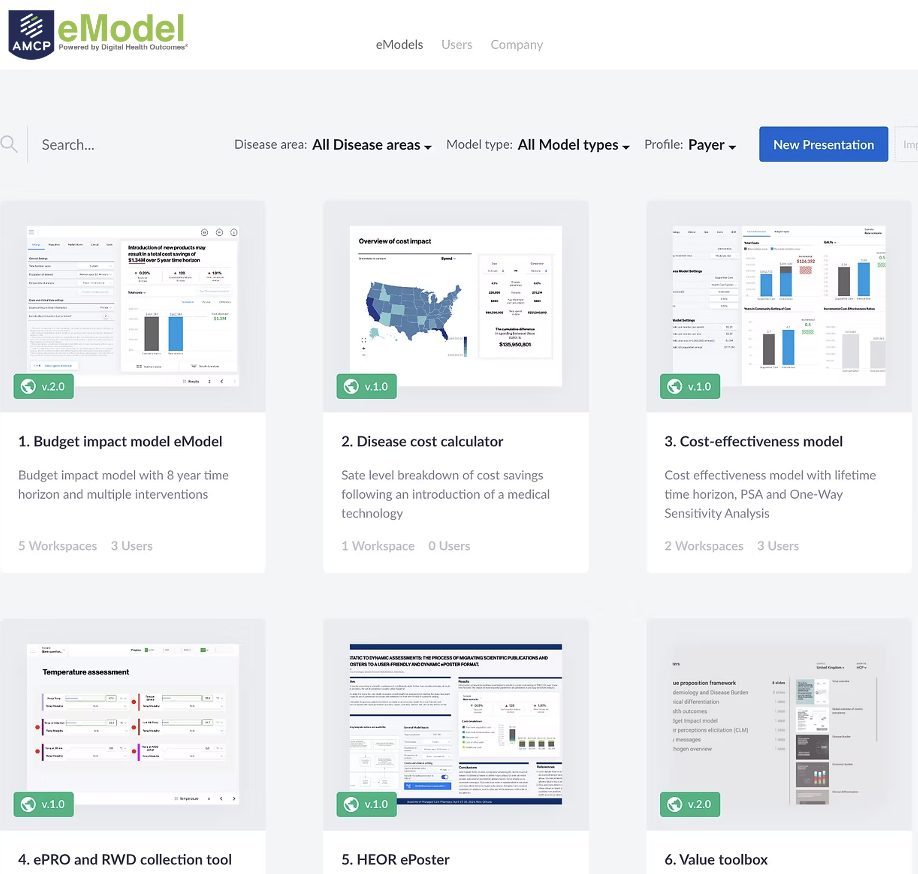
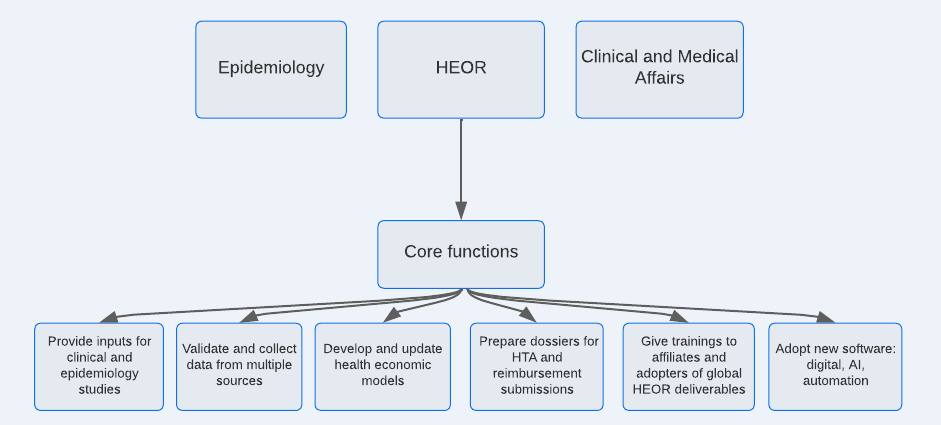
The figure below presents core HEOR strategy functions within pharmaceutical industry
Economic evaluation in HEOR — Basic methods
HEOR economic evaluations compare the costs and effectiveness of different medicines with similar indications or treatment protocols for a specific disease. This analysis is used not only to create product value but also in negotiations with healthcare decision-makers.
There are four main ways of economic evaluation within HEOR:
- Cost-minimization analysis (CMA) – compares the monetary value of treatment costs. It assumes that the clinical outcomes of the products and health interventions being compared are identical. Post-treatment effects are not evaluated.
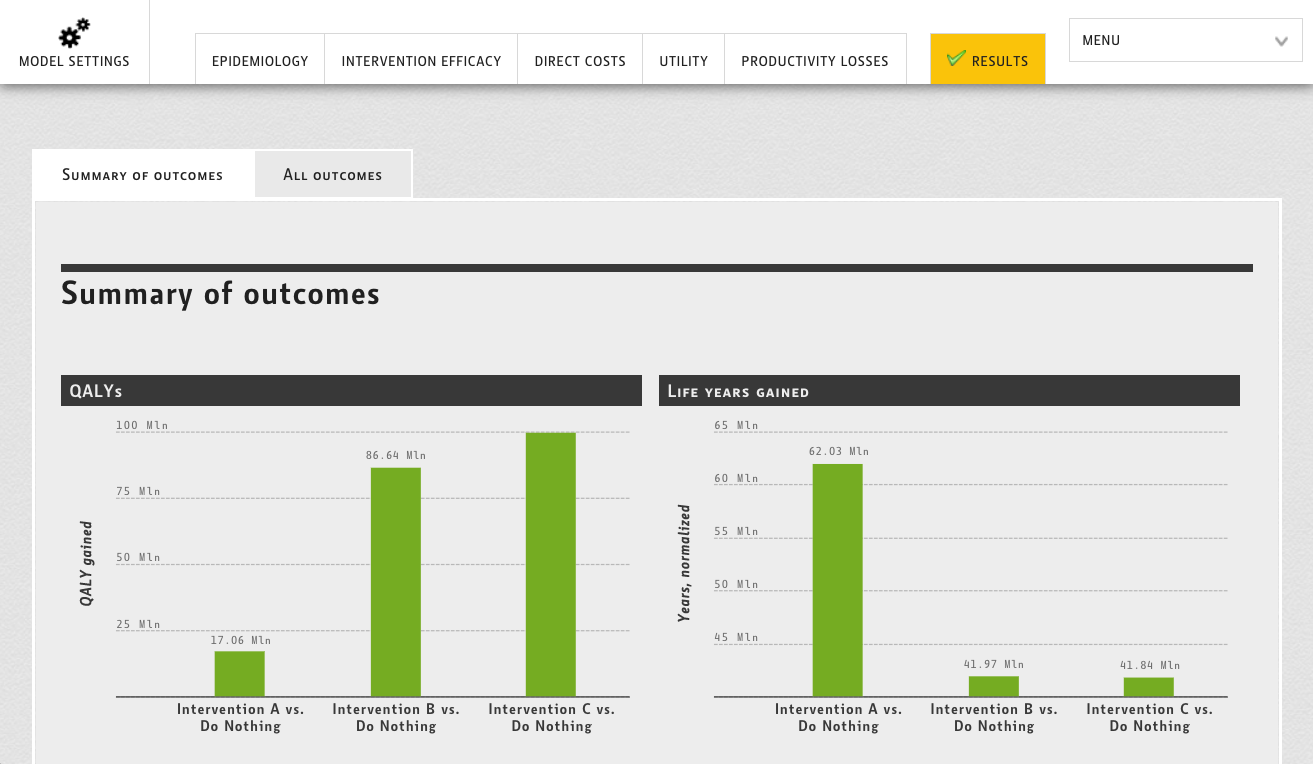
- Cost-effectiveness analysis (CEA) – compares the monetary value of costs to clinical outcomes and treatment effects. which are measured in different units, For example number of days without pain or a generic metric like Quality Adjusted Life Years (QALY).
- Cost-utility analysis (CUA) – assessing the monetary cost of drugs or treatment protocols against the overall utility. It also estimates the health benefits expressed in terms of the number of years of life after the course, adjusted for quality.
- Cost-benefit analysis (CBA) – comparing the monetary value of treatment costs and the effects of treatment, also expressed in monetary terms.
What data does HEOR use?
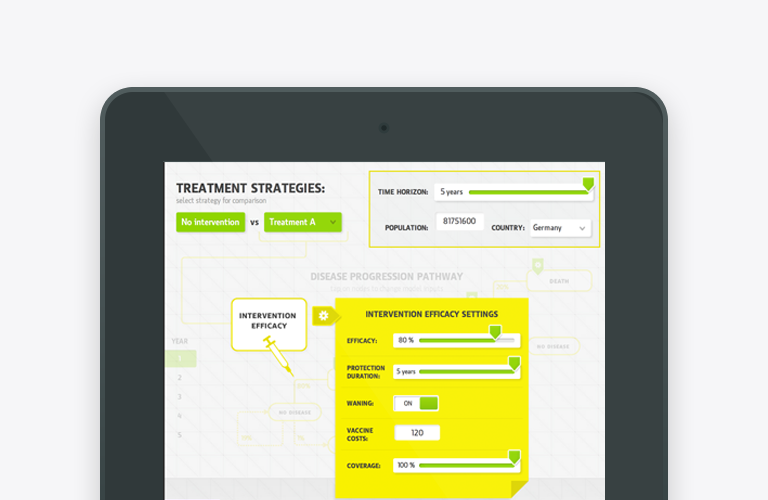
Health Economics evaluations and Real World Evidence (RWE) studies take disaggregated data from multiple sources to produce estimates of cost-effectiveness and budget impact based on expected clinical outcomes projected over a specific time horizon with Markov model. Other types of health economic models, such as budget impact model can be used to produce a more precise projection of expected costs over the 5 year time horizon.
The following data is mostly used:
- Incidence and prevalence rates of the disease.
- Difference in clinical effect (efficacy, effectiveness).
- Direct and Indirect Costs of the disease.
- Quality of Life measures (normative utility and disutility).
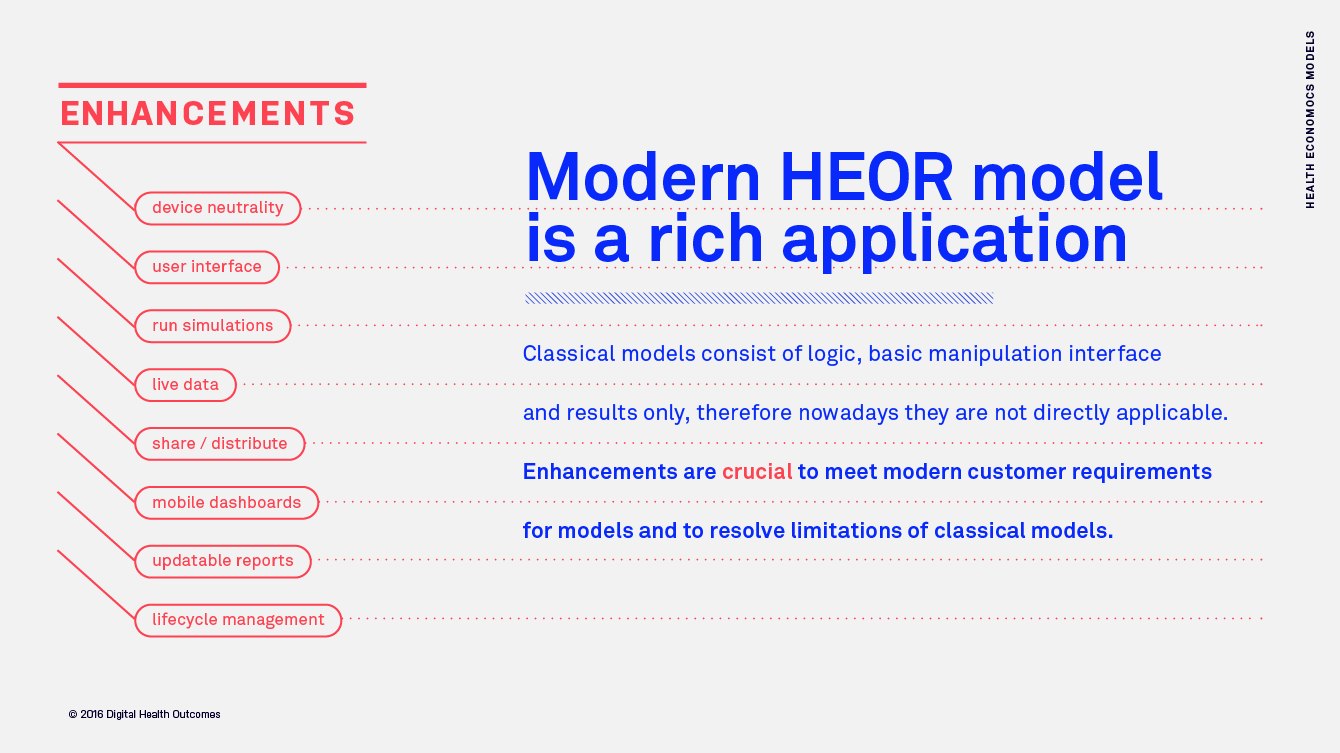
Value of data processing innovations in HEOR modelling
In this article we describe technology innovations for seamless data updates of health economics models. We also explore the benefits of real-time dynamic data linked to HEOR cost-effectiveness and budget impact models.
At least every year a health economics model has to be updated with new cost data to produce actual estimates for the new fiscal year. While it is quite straightforward to update the model with new price data, spotting and related integration of new epidemiological or clinical evidence, including the one published by other manufacturers and HEOR research centres might not be that easy and fast. Very often multiple specialists, opinion leaders and organisations (industry, academia, consulting) are involved in economic evaluations of blockbuster pharmaceutical and medical device innovations. There is an anticipated time lag of about 1 to 3 years, between acceptance of new evidence, related updates of global cost-effectiveness models, updates of local cost-effectiveness models and decisions taken by a healthcare payer.
Researchers putting up HEOR based on evidence and data synthesis require a single environment that connects all dots together: data, models, and reports to produce dynamic and real-time estimates of cost-effectiveness of drugs and healthcare technologies.
The anticipated monetary, health and societal losses due to delays in cost-effectiveness and budget impact model updates could be huge. Governments and healthcare purchasers spend millions to manage different health conditions with an available portfolio of healthcare technologies. Can digital innovation suggest the next best treatment option to choose? At least to some extent, by connecting live input data feeds to economic models and providing a single ecosystem for review of changes made, activities and data owners. Live data feeds and automated data update suggestions could significantly reduce the time required to make an update to the HEOR models and to make the final decision.
You can also find more HEOR resources (good practices reports, guidelines, and others) on the ISPOR web page.
Digital Health Outcomes (DHO) provides a software solution for HEOR models (budget impact and cost-effectiveness) digitisation. DHO designs the overall information architecture and finds technical ways to stream data from multiple sources into interactive web based HEOR models. To better understand the value of health economics models migration to web or mobile (iPad), please see our recent blog article.