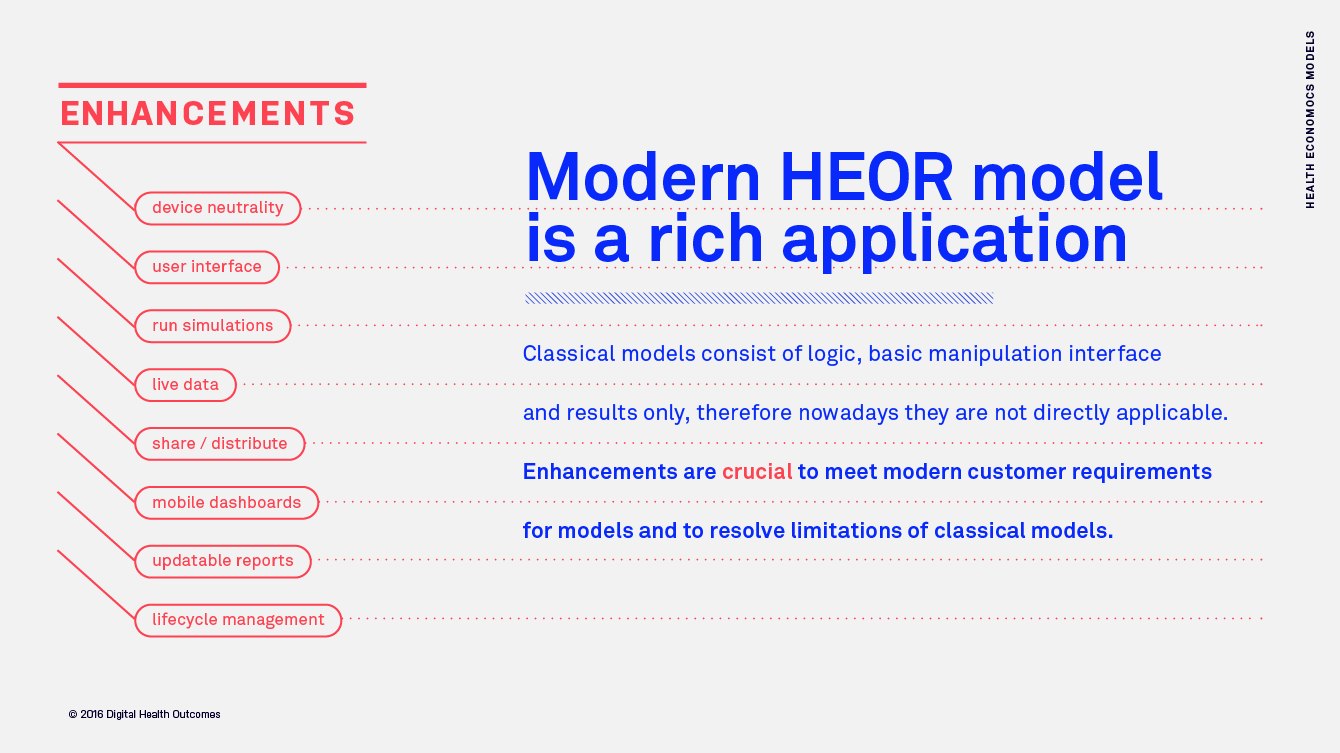
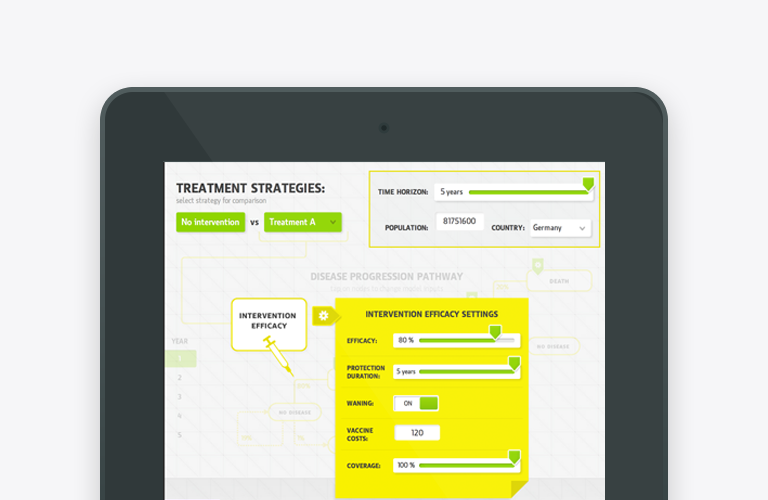
Health Economics
Much faster probabilistic sensitivity analysis (PSA) with cloud based horizontal autoscaling
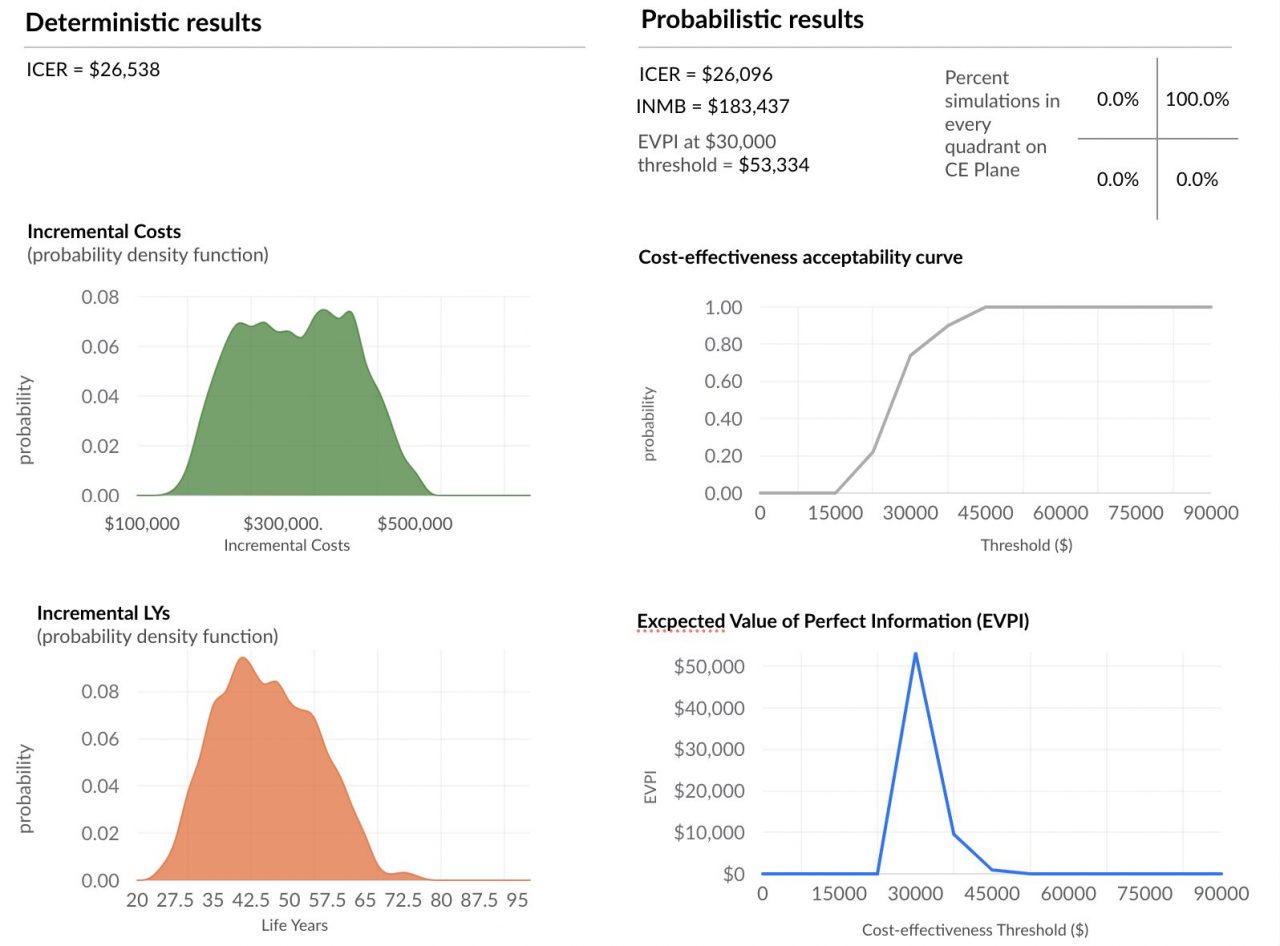
Background and definitions: Decision-makers require estimates of decision uncertainty alongside expected net benefits (NB) of interventions. This requirement may be difficult in computationally expensive models, for example, those employing patient level simulation[1]. Probabilistic sensitivity analysis (PSA) is… Read more
Automatic Data Sourcing for Health Economic Models – Interoperable data for timely decision-making
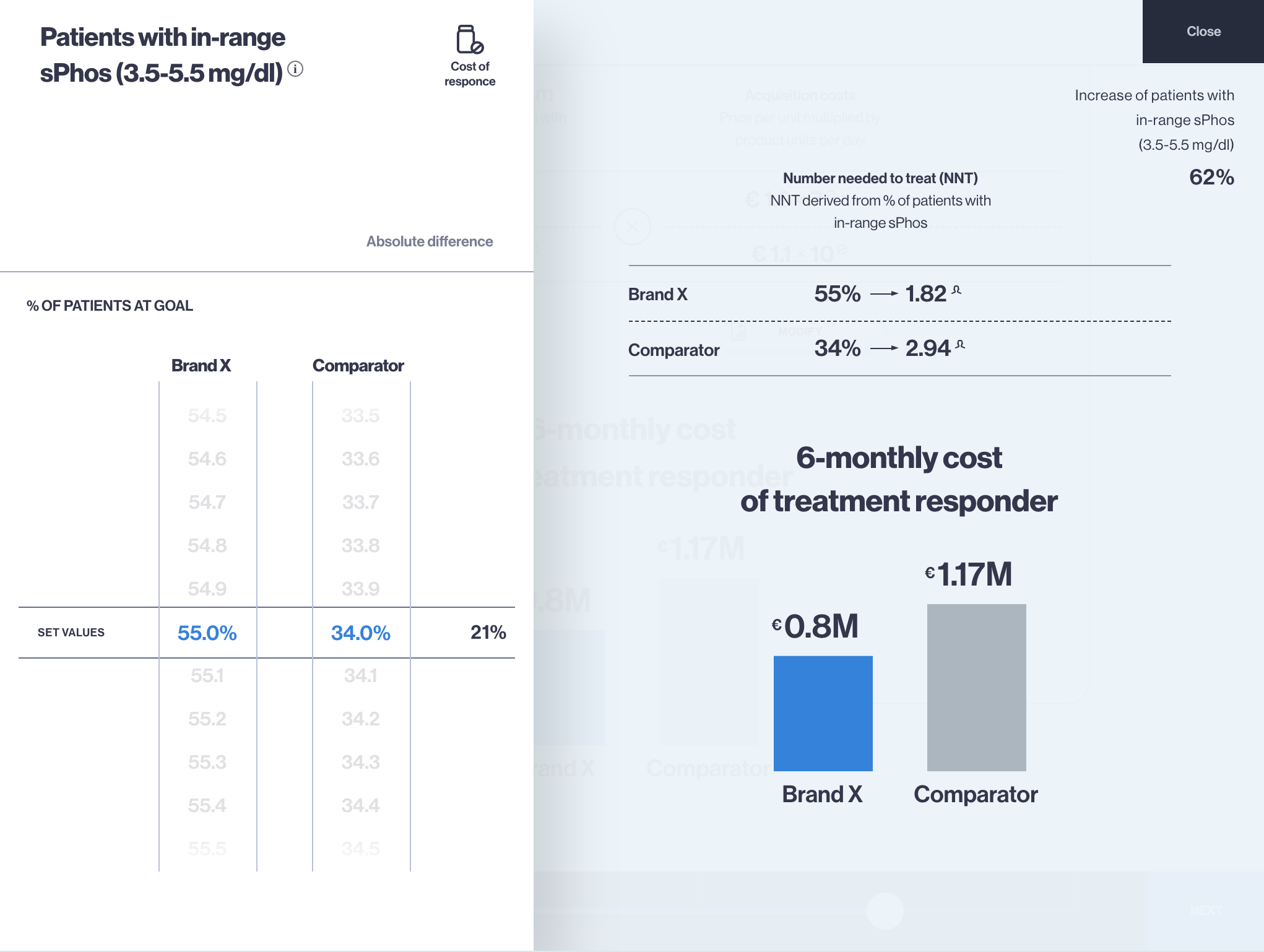
This article is inspired by the exciting idea of data interoperability in health economics modelling process, which means sourcing (fetching) inputs for models in real-time, using modern software infrastructure capabilities. On a larger scale, development of… Read more
Digital Health Outcomes launched interactive health economic modeling platform together with the Institute for Clinical and Economic review (ICER).
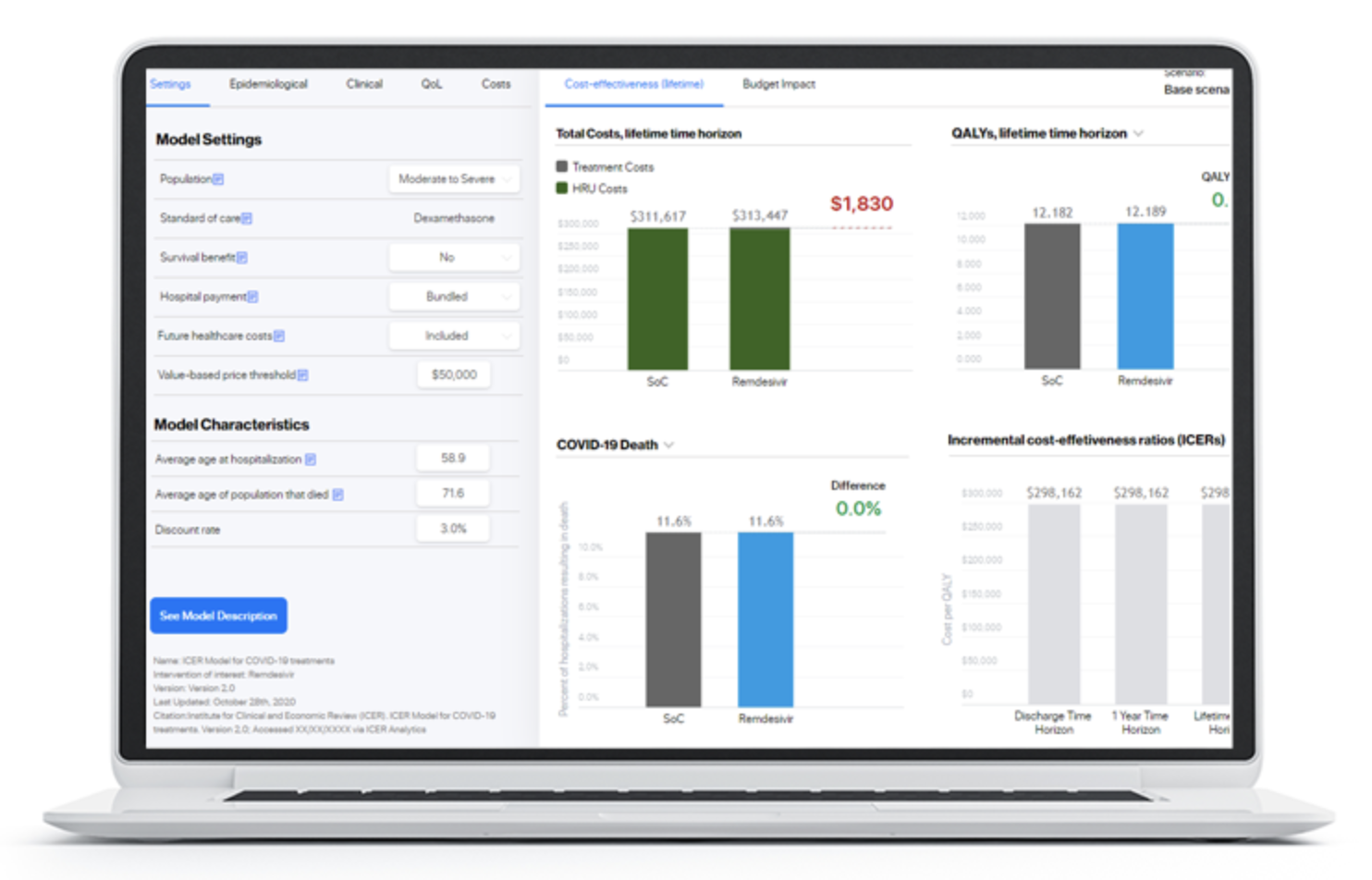
We are delighted to support the US based Institute for Clinical and Economic (ICER) in launching a dedicated interactive modeling platform hosting a wide variety of cost-effectiveness and budget impact models developed by ICER and their… Read more
Systematic Review and Meta-Analysis — Overview
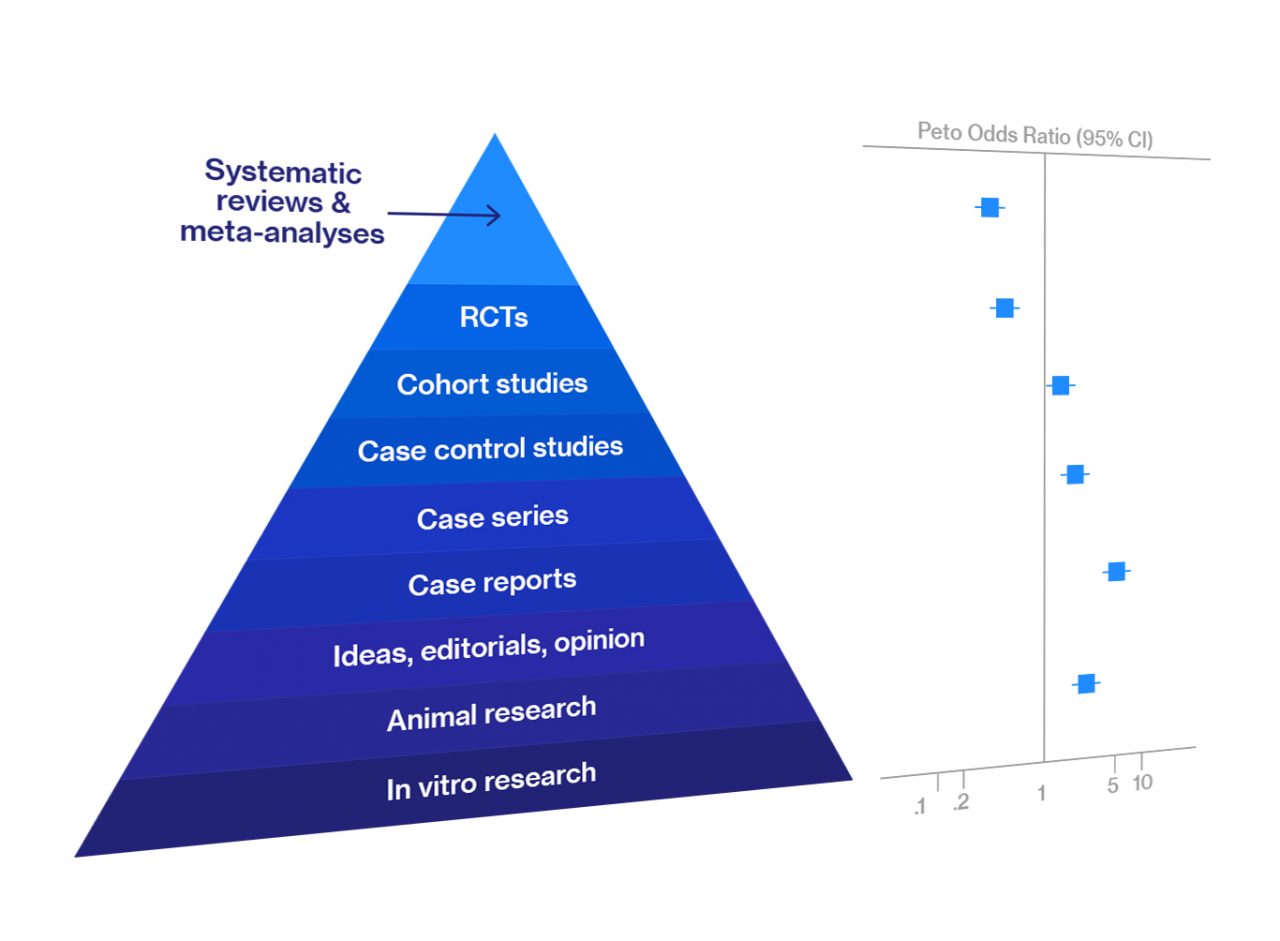
Meta-analysis and systematic review are at the top of the hierarchy of evidence for data reliability in various types of biomedical research. They are an integral part of evidence-based medicine. Most clinicians begin searching for the best, evidence-based… Read more
Cost-Effectiveness Analysis VS Cost-Benefit Analysis
Before investing in a pharmaceutical product or a medical device company, a payer must evaluate the economic value of the proposed intervention. Hence, it’s important to understand modelling methods used to demonstrate the value and the… Read more
What do you need to know about Health Economics and Outcomes Research (HEOR) in Pharma
HEOR or Health Economics and Outcomes Research – term and job position/function used in the pharmaceutical industry. It is also an emerging field of research in health economics that focuses on providing pharmaceutical and life sciences companies… Read more