"In international trade, market access refers to a company's ability to enter a foreign market by selling its goods and services in another country." [1]
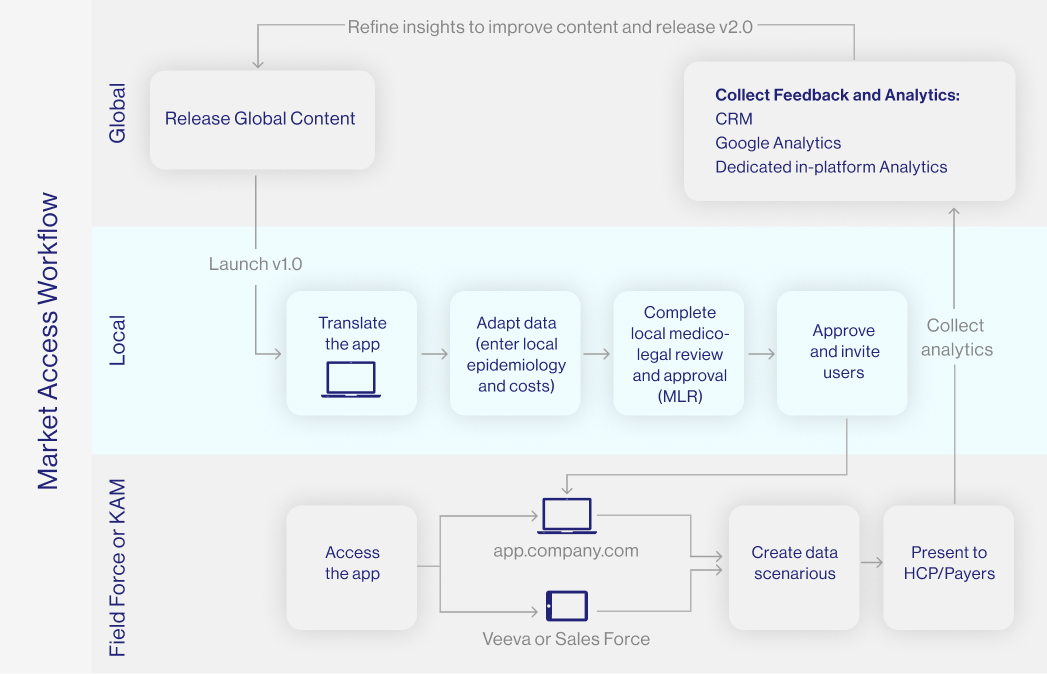
Market access in the pharmaceutical industry means the ability for a medical product to be available for sale to end users, such as patients, physicians, or hospitals. It also means that the product is reimbursed either fully or partially by the payer.
"Pharmaceutical spending in the United States, Canada, and the EU is growing. Public payers cover a large portion of these costs and have responded by instituting various pricing and access policies to limit their expenditure." [2]
In order for a medical drug to be allowed to be marketed, it must go through a complex and time-consuming process of registration and approval from regulatory agencies, such as the FDA in the United States or the EMA in the European Union. Once approved for use in the EU each country's HTA body will then negotiate on price and conditions for market entry.
Market access consultancy in the pharmaceutical industry is critical to the success of the drug and the manufacturing company because it facilitates the ability to better design effective commercialisation and communication pathways for patient access to healthcare products.